How To Find Limiting Reagent With Moles
Determine the balanced chemical equation for the chemical reaction. Reactions occur on the molecular scale so I begin by converting my 100 g of PbNO 3 2 to moles of PbNO 3 2.

Using A Quadratic Equation To Find Equilibrium Moles Given Kp Question 4 A Level Chemistry Tutor Youtube In 2021 Quadratics Quadratic Equation Tutor
Showing how to find the limiting reagent of a reaction.

How to find limiting reagent with moles. As they move through the book students will experience traditional organic reactions. If we divide our moles of H 2 into moles of N 2 our value will tell us which reactant will come up short. Rock Chalk Jayhawk KU.
The one that produces the least amount of the end product is the limiting reagent. Remember to use the molar ratio between the limiting reactant and the product. Then convert all the given information into moles by using molar mass as a conversion factor.
The reaction between NH 3 and O 2 yields NO nitric oxide and H 2 O water. Convert the number of moles of product to mass of product. Any value greater than the above ratio means the top reactant is in excess to the lower number.
Find the limiting reactant by looking at the number of moles of each reactant. The next step is to calculate the mole ratio from the given information. Determine the number of moles of each reactant.
Calculate the molecular weight of each reactant and product 3. By the way did you notice that I bolded the technique to find the limiting reagent. The reactant that would produce the smallest amount of product is the limiting reagent.
Convert all amounts of reactants and products into moles 4. A value less than the ratio means the top reactant is the limiting reactant. Using the mole ration Using the product approach In order to calculate the mass of the product first write the balanced equation and find out which reagent is in excess.
Finding the limiting reagent by looking at the number of moles of every reactant. Ill begin with the first question. If we only have x moles of a reactant we can only expect y moles of product.
Write a balanced equation for the reaction 2. Approach 1 The Reactant Mole Ratio Method. It limits the amount of PbI 2 I can form and so it is the limiting reagent.
Figure out the limiting reagent. The limiting reactant or reagent can be determined by two methods. The key is to keep the same reactant on top as the step above.
In a given stoichiometry problem you will use this reactant to determine amount of product formed. A video made by a student for a student. To identify the limiting reactant calculate the number of moles of each reactant present and compare this ratio to the mole ratio of the reactants in the balanced chemical equation.
First determine the balanced chemical equation for the given chemical reaction. How To Get Limiting Reagent From Moles experiments designed to utilize microscale glassware and equipment demonstrate the relationship between organic chemistry and everyday life with project-and biological or health science focused experiments. I can next use the chemical reaction to determine the number of moles of PbI 2 produced from this amount of PbNO 3 2.
A If the calculated MOLES NEEDED is greater than the MOLES HAVE for a given reactant then that reactant is the limiting reagent. If reactant B is the limiting reagent moles of B left over on completion will be 0 n B 0 mol The reactant in excess is the reactant that is not completely used up during the chemical reaction that is there is some of this reactant left over. Convert all given information into moles most likely through the use of molar mass as a conversion factor.
Calculate the number of moles of product that can be obtained from the limiting reactant. Youre going to need that technique so remember it. Using Mole Ratios Lets apply this method to the reaction of ammonia NH 3 and molecular oxygen O 2 to figure out the limiting reactant of the two.
The steps to determine the limiting reagent or the limiting reactant is as follows. To find out the limiting reagent you need to find the amount of product that can be made with respect to each reactant involved. The substance that has the smallest answer is the limiting reagent.
Compare the mole ratio of the reactants with the ratio in the balanced chemical equation to determine which reactant is limiting. To find the limiting reagent take the moles of each substance and divide it by its coefficient in the balanced equation.

Stoichiometry Limiting Reagent Stoichiometry Chemistry Chemistry School Help

What Is Stoichiometry And Why Is It Used In Chemistry A Plus Topper Whatisstoichiometry Stoichiometry Chemistry Chemistry Chemistry Worksheets

How To Solve Stoichiometry Problems With A Conversion Box The How To How To Videos Watch And Learn Chemistry Worksheets Teaching Chemistry Chemistry Help

Limiting Reactant Practice Problem Science Sciencewithtylerdewitt Tylerdewitt Tutor Sciencehelp School Help Online School Chemistry

How To Calculate An Empirical Formula Chemistry Worksheets Teaching Chemistry Chemistry Help

Nscc Alp Chem1047 Feb 10 2015 12 12 Pm Ideal Gas Law Molar Mass Chemistry

Need To Calculate The Limiting Reactant Of A Chemical Reaction Chemical Reactions Chemical Chemical Equation

Stoichiometry Limiting Reagent Ice Box Madison Nowlin Ice Box Chemistry Class Chemistry

How To Calculate Percent Yield In Chemistry Teaching Chemistry Chemistry Physical Chemistry
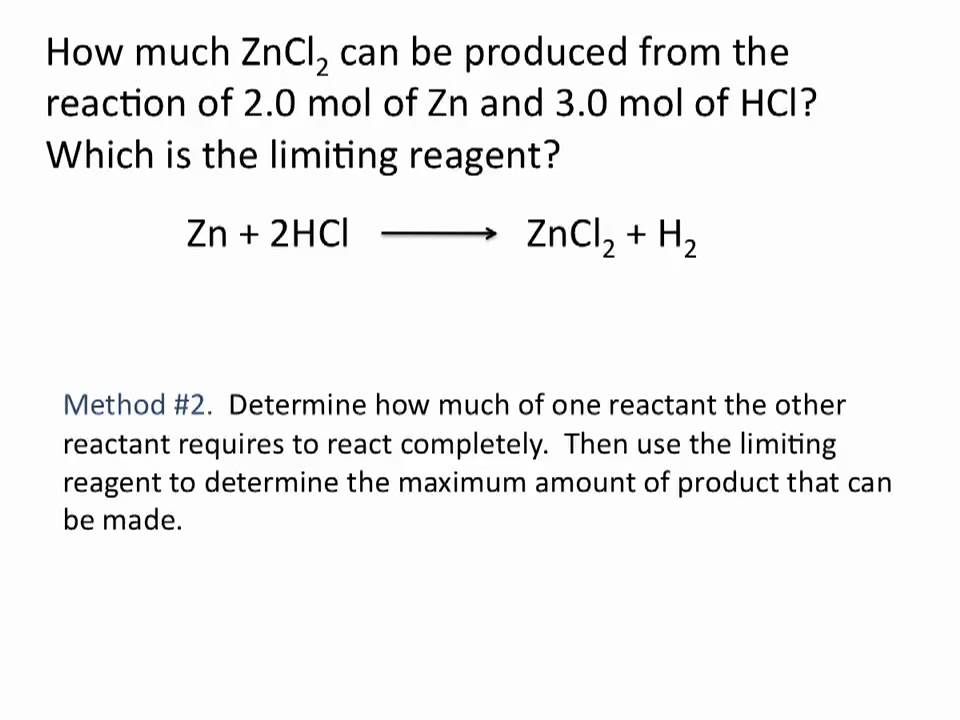
Limiting Reagent Chemistry Tutorial Youtube Chemistry Tutorial School
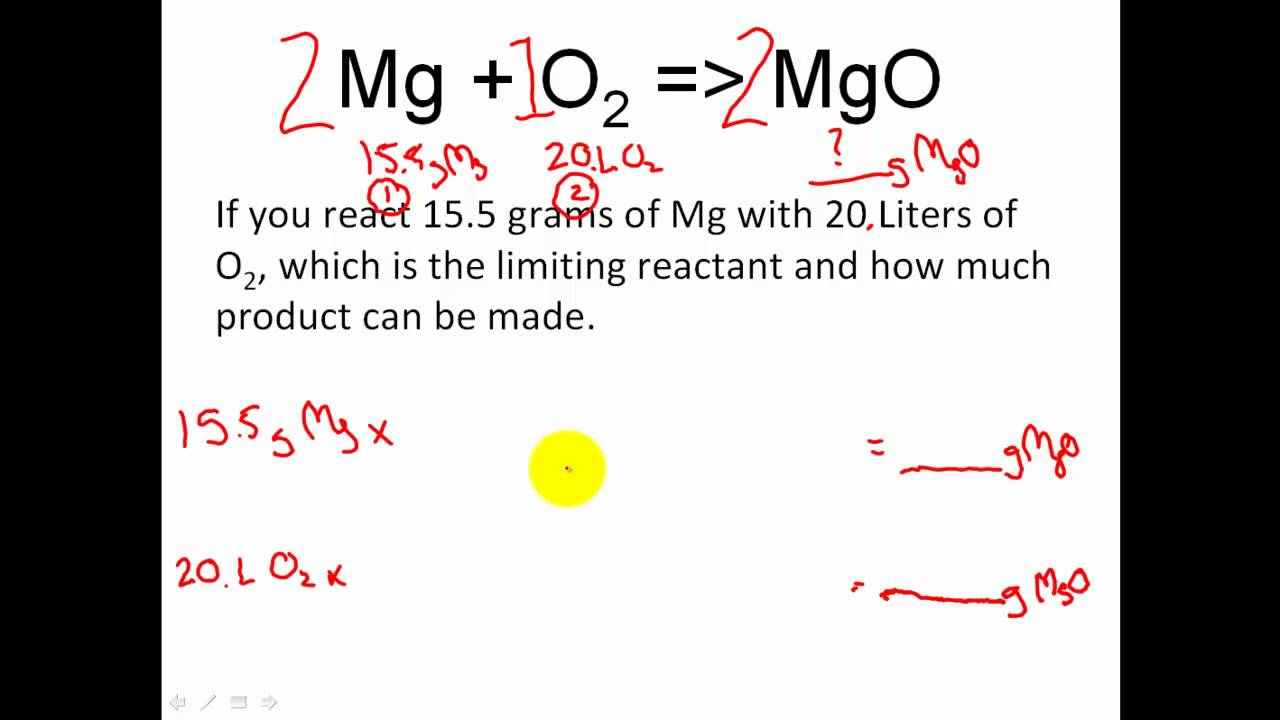
Stoichiometry Limiting Reactant Excess Reactant Stoichiometry Moles Module 6 Learning Psychology Apologia Chemistry School Work

How To Find The Mole Ratio And Molar Mass Youtube

Limiting Reactants And Percent Yield Apologia Chemistry Chemistry Chemistry Class

Limiting Reagents Grams To Grams Teaching Science Chemistry Teaching

Limiting Reactant Easy Science Easy Science Understanding Teaching

Finding Limiting And Excess Reagents Chemistry Math Equations Excess



Post a Comment for "How To Find Limiting Reagent With Moles"