How To Find Limiting Reagent With Volume
Then use each molar mass to convert from mass to moles. So if you find that you have 100 mol of both A and B you know that A is the limiting reactant as for one mole of B to react completely you would need 2 moles of A and since the ratio is 21 with the 1 mole of A that you have you only need 05 mol of B showing that A is the limiting reactant and that B will be in excess at the end of the reaction.

Using A Quadratic Equation To Find Equilibrium Moles Given Kp Question 4 A Level Chemistry Tutor Youtube In 2021 Quadratics Quadratic Equation Tutor
Then use each molar mass to convert from mass to moles.

How to find limiting reagent with volume. After identifying the limiting reactant use mole ratios based on the number of moles of limiting reactant to determine the number of moles of product. One method is to find and compare the mole ratio of the reactants used in the reaction approach 1. The limiting reagent or reactant in a reaction is found by calculating the amount of product produced by each reactant.
Use the given densities to convert from volume to mass. Limiting Reagents and Reactants in Excess Example. The reactant that produces the least amount of product is the limiting reactant.
It limits the reaction from continuing because there is none left to react with the in-excess reactant. Write a balanced equation for the reaction 2. The solid or liquid matter has a definite and constant volume It can be measured by various methods.
Since equal volumes of equal concentration solutions will have equal numbers of moles of each reactant it follows that the solution that has the lower concentration will act as a limiting reagent. So remember when you add equal volumes of each reactant their respective molarities will determine which one acts as a limiting reagent and which one is in excess. A chemical equation is like a recipe for a reaction so it displays all the ingredients or terms of a chemical reaction.
It includes the elements molecules or ions in the reactants and in the products as well as their states and the proportion for how much of each particle is create relative to one another through the stoichiometric coefficient. After identifying the limiting reactant use mole ratios based on the number of moles of limiting reactant to determine the number of moles of product. Using mole ratios determine which substance is the limiting reactant.
Use the given densities to convert from volume to mass. Convert all amounts of reactants and products into moles 4. The mole and volume of gases.
Find the limiting reagent and the reactant in excess when 100 mL of 02 mol L-1 NaOH aqueous solution react completely with 50 mL of 05 mol L-1 H 2 SO 4 aqueous solution. About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators. Write the balanced chemical equation for the chemical reaction.
Concentration and volume of solutions given. Practice Problems To practice making solutions of particular concentrations solve the following problems. Remember to keep all units of volume and mass the same or your results will be incorrect.
There are two ways to determine the limiting reagent. Examples have been color coded to make it easier to see which variable corresponds to which. Limiting reactant is the reactant which is completely consumed during the chemical reaction and reacts with other reactants to produce the lowest number of moles of the product.
The limiting reagent is the one that is totally consumed. Figure out the limiting reagent. Using mole ratios determine which substance is the limiting reactant.
Calculate the molecular weight of each reactant and product 3. Mixing Reagents of a Desired. Reactants to Products.

Percent Error Worksheet Answer Key Fresh Percent Yield Worksheet Super Teacher Worksheets Chemistry Worksheets Chemistry

Chemistry Mr Causey Shows How To Use Ideal Gas Law To Find Volume Teaching Chemistry Science Chemistry Chemistry Classroom

Limiting Reactant Easy Science Easy Science Understanding Teaching

Need To Calculate The Limiting Reactant Of A Chemical Reaction Chemical Reactions Chemical Chemical Equation

Limiting Reactant Practice Problem Science Sciencewithtylerdewitt Tylerdewitt Tutor Sciencehelp School Help Online School Chemistry
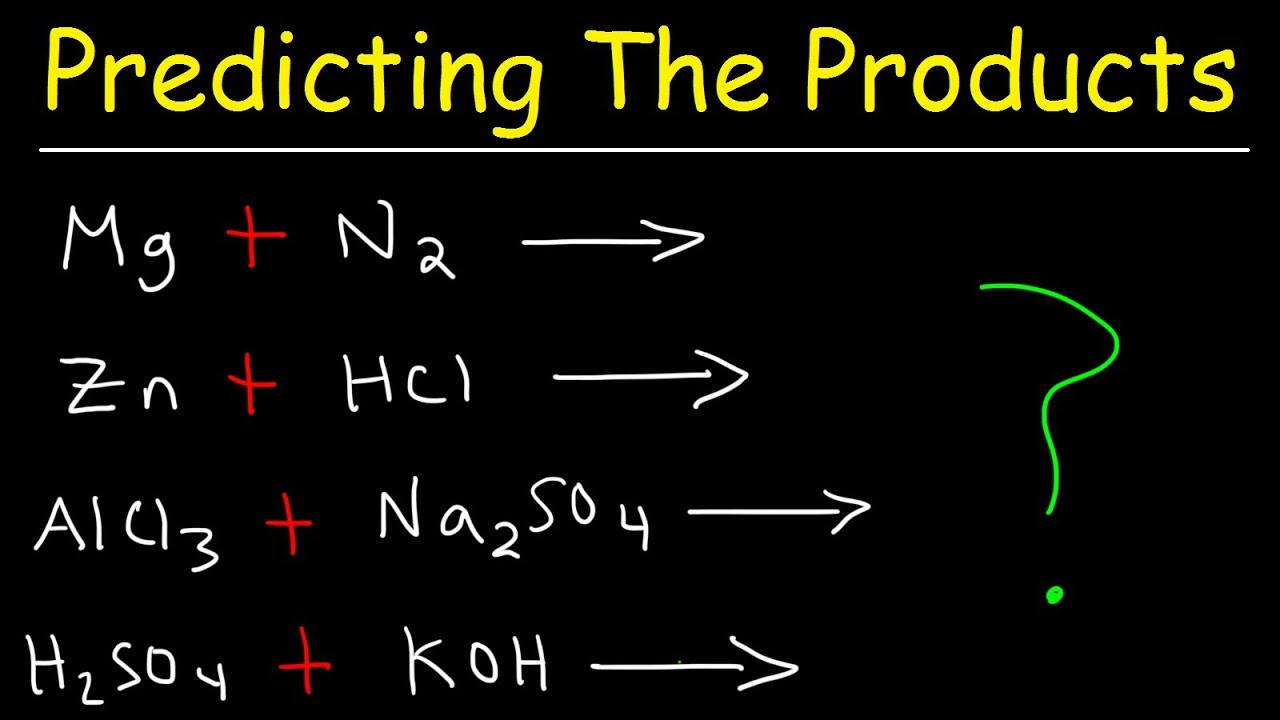
Predicting The Products Of Chemical Reactions Chemistry Examples And P Chemistry Worksheets Persuasive Writing Prompts Apologia Physical Science

Limiting Reactant Limiting Reactant Problems Chemistry Chemical Equation Ap Chem

Finding Limiting And Excess Reagents Chemistry Math Equations Excess

Chemistry 12 3 Limiting Reagent And Percent Yield Chemistry Percents Pure Products

Theoretical Yield Chemistry Chemical Reactions Find Percentage
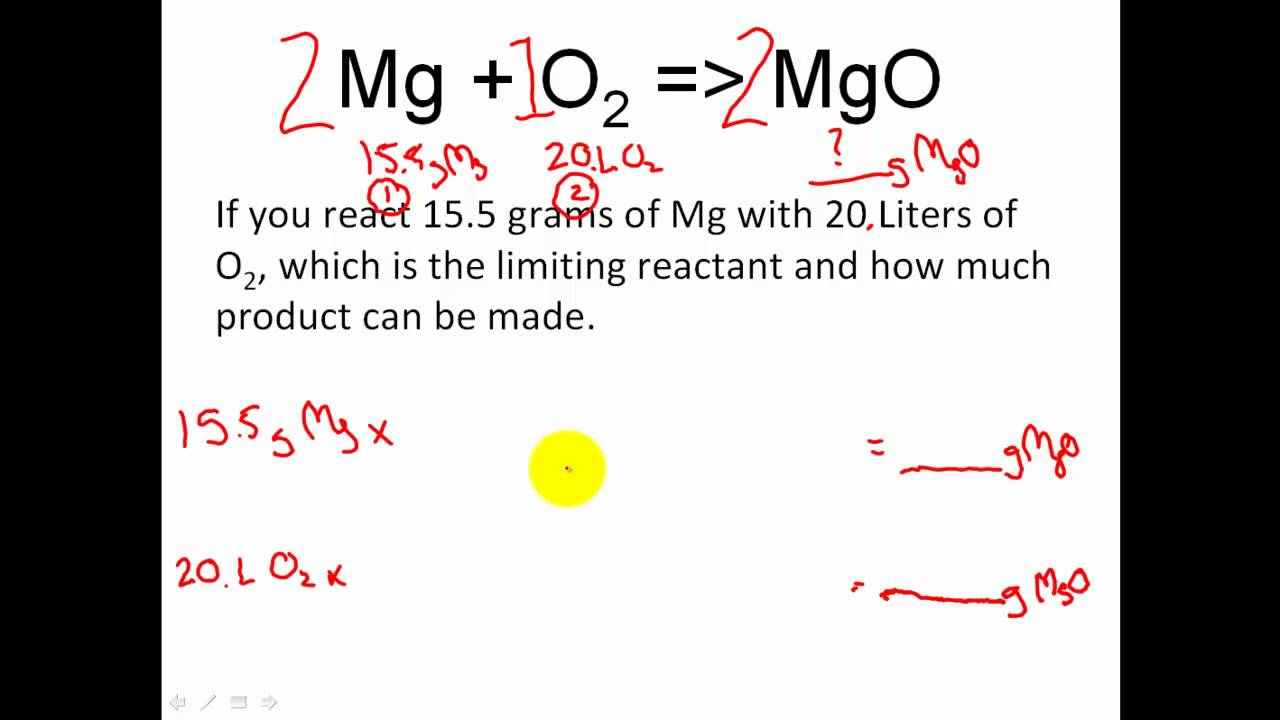
Stoichiometry Limiting Reactant Excess Reactant Stoichiometry Moles Module 6 Learning Psychology Apologia Chemistry School Work

Reversible Reaction Easy Science Easy Science Understanding Reactions

Stoichiometry Limiting Reagent Stoichiometry Chemistry Chemistry School Help

Nscc Alp Chem1047 Feb 10 2015 12 12 Pm Ideal Gas Law Molar Mass Chemistry

Limiting Reactant With Numerical Example Complete Lecture Fsc 11th C 11th Chemistry Chemistry Lecture

High School Science Learning Activity Limiting Reactant Learning Liftoff High School Science Learning Science Teaching Chemistry

Stoichiometry Limiting Reactant Of A Reaction Chemistry Teaching Chemistry Science Chemistry Chemistry Classroom
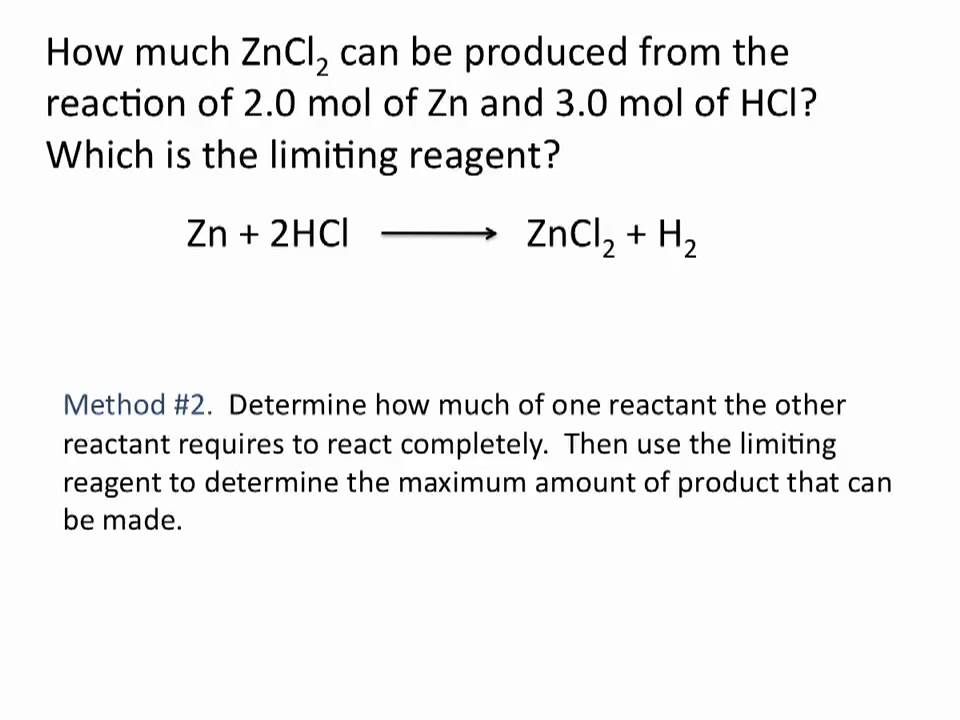
Limiting Reagent Chemistry Tutorial Youtube Chemistry Tutorial School

Post a Comment for "How To Find Limiting Reagent With Volume"